 Pain management is a growing story at CES 2017. I covered the topic of Sleep and Pain at CES one year ago in the Huffington Post, and this year, the category has grown in both number of innovations and mode of pain management. At CES 2017, there were exhibitors of FDA-approved devices, sleep-and-pain focused tech, wristbands for relaxation and nausea-management, and a $5,999 device for calming meditation that’s being used in addiction programs.
Pain management is a growing story at CES 2017. I covered the topic of Sleep and Pain at CES one year ago in the Huffington Post, and this year, the category has grown in both number of innovations and mode of pain management. At CES 2017, there were exhibitors of FDA-approved devices, sleep-and-pain focused tech, wristbands for relaxation and nausea-management, and a $5,999 device for calming meditation that’s being used in addiction programs.
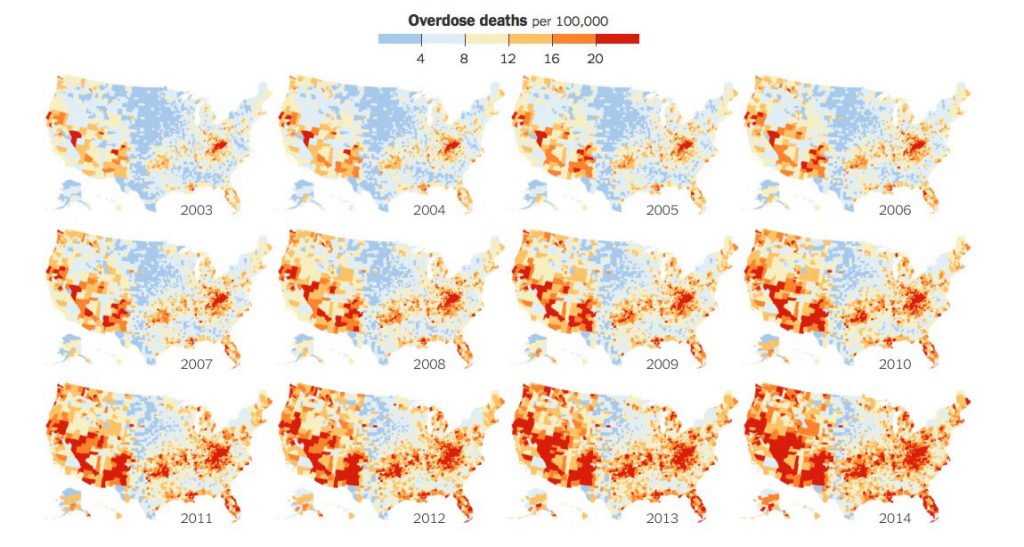
What’s driving growth and acceptance of this health tech segment is the opioid crisis which has become a public health epidemic across the U.S. The maps with increasing orange color illustrate the growing epidemic of deaths by overdose in the U.S. to 2014.
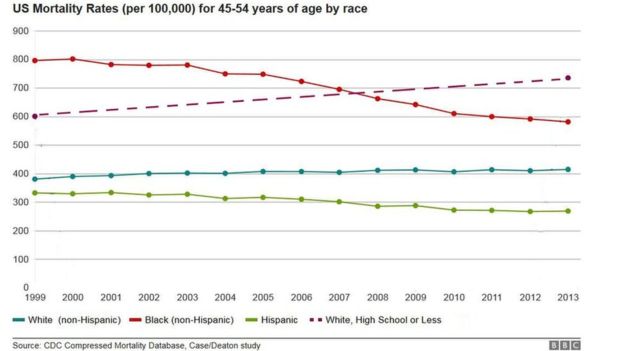 The raw statistic is that dozens of people die everyday due to drug overdose. Underneath that number is the fact that the mortality rate for white men in America stopped declining due to what Anne Case of Princeton University has called, “deaths of despair.” A contributor to the flatlining of mortality declines for white males is the dramatically growing use of opioids and other drugs in the post-Recession era. See the rising purple line in the graph for the visual of this sobering data point. What’s more interesting is the use of alternatives to opioids for pain relief, the most interesting in the class being kratom. Kratom is a plant native to Thailand that has been shown in lab tests to provide a smoother, more long lasting relief of pain, without having as many of the habit forming and addictive properties that prescription painkillers carry.
The raw statistic is that dozens of people die everyday due to drug overdose. Underneath that number is the fact that the mortality rate for white men in America stopped declining due to what Anne Case of Princeton University has called, “deaths of despair.” A contributor to the flatlining of mortality declines for white males is the dramatically growing use of opioids and other drugs in the post-Recession era. See the rising purple line in the graph for the visual of this sobering data point. What’s more interesting is the use of alternatives to opioids for pain relief, the most interesting in the class being kratom. Kratom is a plant native to Thailand that has been shown in lab tests to provide a smoother, more long lasting relief of pain, without having as many of the habit forming and addictive properties that prescription painkillers carry.
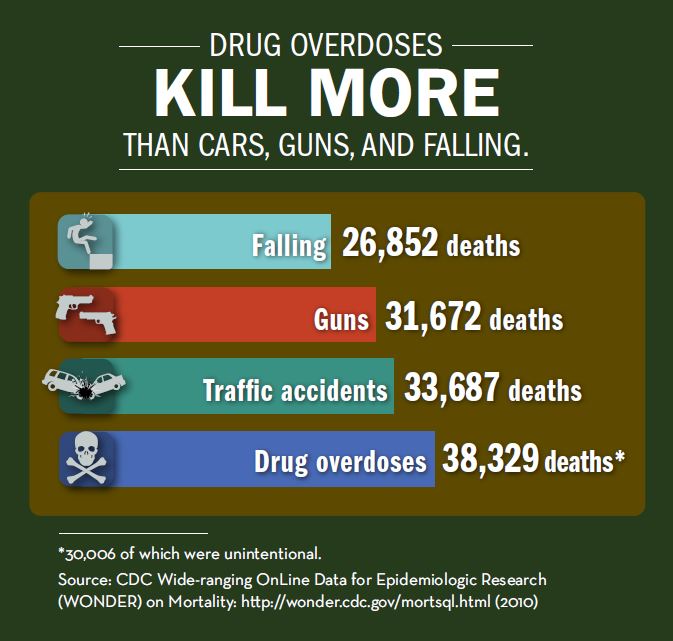
“Chronic pain not caused by cancer is among the most prevalent and debilitating medical conditions but also among the most controversial and complex to manage,” according to Dr. Nora Volkow and Dr. Thomas Mclellan who co-authored a review article on the topic of opioid abuse in chronic pain in the New England Journal of Medicine (March 31, 2016). Over 30% of Americans have some sort of acute or chronic pain, and the prevalence among older adults is over 40%. Opioid analgesics are now the most commonly prescribed class of medications in the U.S., the authors attest.
 The Digital Health Summit track of programming included a session on digital tech for pain called Dying to Get High – Tackling the Opioid Epidemic. The panel included clinician innovators developing digital approaches to managing pain, including Virtual Reality (VR) headsets, digital therapeutics (medications plus mobile health apps), and medications delivered through smart implants.
The Digital Health Summit track of programming included a session on digital tech for pain called Dying to Get High – Tackling the Opioid Epidemic. The panel included clinician innovators developing digital approaches to managing pain, including Virtual Reality (VR) headsets, digital therapeutics (medications plus mobile health apps), and medications delivered through smart implants.
“Codeine, fentanyl, oxycodone, heroin: these are all opioid substances that are fueling the crisis that we have,” Dr Arshya Vahabzadeh, Director of Digital Health at the Harvard Medical School, listed as he introduced the panel.
At CES 2017, several exhibitors featured non-invasive personal health devices that aim to address the pain management challenge. At the most regulated and science-based end of the spectrum of pain-tech options at CES was Quell, developed and marketed by NeuroMetrix. This company’s provenance has been in the FDA regulated medical device space focused on neurology, spinning off from the Harvard-MIT Division of Health Sciences and Technology in 1996. Thus, the company has a long history dealing with pain through neurostimulation in acute care settings. When I met with the company one year ago, they had recently launched the Quell device following FDA approval, an OTC (over-the-counter) consumer-facing medical device that is based on the company’s understanding of pain triggers. The Quell wraps around the calf, regardless of the site of pain (back, hip, knee, wherever). Quell stimulates sensory nerves which then triggers the body’s natural pain relief response. NeuroMetrix’s clinical studies claim that 81% of users reported an improvement in chronic pain.
 Quell was one of several innovations focused on pain at CES 2017. Related devices targeted sleep, with a dozen exhibitors in the CES Sleep Tech Marketplace. Among the sleep tech’s were the Sleep Number 360 AI-smart bed, which won a CES 2017 innovation award; and, the Beddit sleep tracker under-the-sheet bed sensor “with nothing to wear” and integrates with the eClinicalWorks electronic health records system. Another related category was nausea, where the most prominent player in this segment was the ReliefBand, a wristband using neuromodulation to regulate nausea, “retching,” and vomiting, according to its promotions. This FDA-cleared device is being marketed to address nausea due to auto/driving, virtual reality, pregnancy, boating, cruising, trains, flights, gaming, amusements (like Ferris wheel rides), and vertigo. Forbes magazine’s lifestyle section called the ReliefBand one of the “best new travel accessories for the road warrior.”
Quell was one of several innovations focused on pain at CES 2017. Related devices targeted sleep, with a dozen exhibitors in the CES Sleep Tech Marketplace. Among the sleep tech’s were the Sleep Number 360 AI-smart bed, which won a CES 2017 innovation award; and, the Beddit sleep tracker under-the-sheet bed sensor “with nothing to wear” and integrates with the eClinicalWorks electronic health records system. Another related category was nausea, where the most prominent player in this segment was the ReliefBand, a wristband using neuromodulation to regulate nausea, “retching,” and vomiting, according to its promotions. This FDA-cleared device is being marketed to address nausea due to auto/driving, virtual reality, pregnancy, boating, cruising, trains, flights, gaming, amusements (like Ferris wheel rides), and vertigo. Forbes magazine’s lifestyle section called the ReliefBand one of the “best new travel accessories for the road warrior.”
These devices are part of a growing supply side responding to the consumer/patient demand to treat pain without drugs. Dr. Vahabzadeh called the opioid crisis a “doctor and healthcare system-driven crisis.” As evidence-based digital options come to market to deal with this challenge, consumers can take back some power from the healthcare system to reverse this crisis.




 Interviewed live on BNN Bloomberg (Canada) on the market for GLP-1 drugs for weight loss and their impact on both the health care system and consumer goods and services -- notably, food, nutrition, retail health, gyms, and other sectors.
Interviewed live on BNN Bloomberg (Canada) on the market for GLP-1 drugs for weight loss and their impact on both the health care system and consumer goods and services -- notably, food, nutrition, retail health, gyms, and other sectors. Thank you, Feedspot, for
Thank you, Feedspot, for  As you may know, I have been splitting work- and living-time between the U.S. and the E.U., most recently living in and working from Brussels. In the month of September 2024, I'll be splitting time between London and other parts of the U.K., and Italy where I'll be working with clients on consumer health, self-care and home care focused on food-as-medicine, digital health, business and scenario planning for the future...
As you may know, I have been splitting work- and living-time between the U.S. and the E.U., most recently living in and working from Brussels. In the month of September 2024, I'll be splitting time between London and other parts of the U.K., and Italy where I'll be working with clients on consumer health, self-care and home care focused on food-as-medicine, digital health, business and scenario planning for the future...