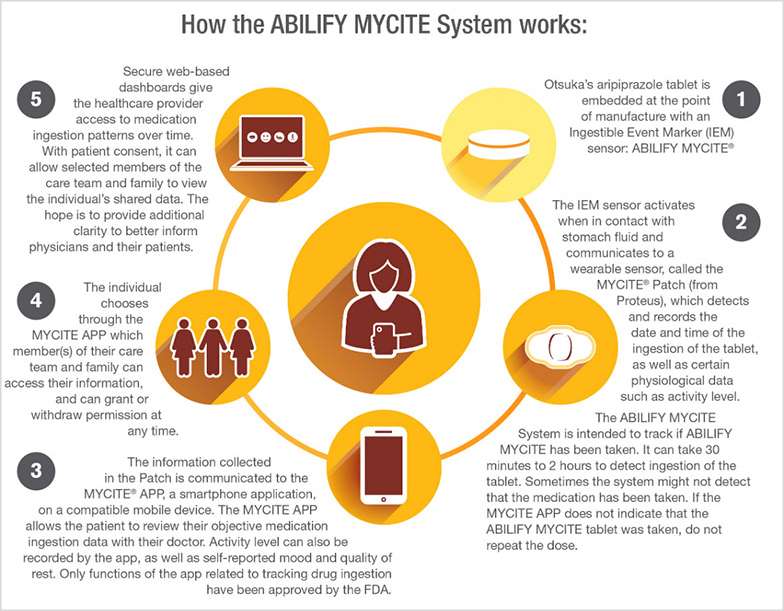
 Yesterday, the FDA approved a “digital ingestion tracking system,” the first drug in the U.S. that has an ingestible (in other words, safely edible) sensor built into the pill. That sensor tracks that the medication was taken, which helps with adherence, meant to help ensure that patients who are prescribed the medicine do indeed take the regimen as prescribed. Once ingested, the sensor in the pill communicates to a wearable patch on the patient that then communicates information to a mobile health app that tracks the pill-taking via smartphone. Patients can allow their family and clinicians access to that information via a web portal.
Yesterday, the FDA approved a “digital ingestion tracking system,” the first drug in the U.S. that has an ingestible (in other words, safely edible) sensor built into the pill. That sensor tracks that the medication was taken, which helps with adherence, meant to help ensure that patients who are prescribed the medicine do indeed take the regimen as prescribed. Once ingested, the sensor in the pill communicates to a wearable patch on the patient that then communicates information to a mobile health app that tracks the pill-taking via smartphone. Patients can allow their family and clinicians access to that information via a web portal.
This digital therapeutic product covers Abilify MyCite, a medication that treats schizophrenia and episodes associates with bipolar I disorder, along with being used as a complementary treatment for depression in adults.
It took two organizations in partnership to bring this innovation to market. Proteus is the developer of the MyCite platform technology — the patch and the app. Otsuka markets Abilify, which according to its label is an add-on treatment for adults with depression when an antidepressant is not enough; treats manic or mixed episodes associated with bipolar I disorder in adults and some pediatric patients; treats schizophrenia in adults and some adolescents; and, treats irritability associated with certain patients on the autism spectrum.
This alliance expands the digital health landscape beyond mobile apps, medical devices, and remote health monitors. In fact, this technology system encompasses all three of these aspects.
Here’s a link to Otsuka and Proteus’s combined press release on this historic event for a “digital medical system,” as the announcement calls it.
Health Populi’s Hot Points: Think of the Abilify-MyCite approval as a milestone in moving up the adoption S-curve of digital therapeutics, now that the U.S. federal regulator, the FDA, has approved the technology for human medical use.
Furthermore, given the form factor of a pill+sensor, we can consider this part of the larger Internet of Things for healthcare, with a pill being “the Thing” that is connected to the Internet via the ingestible sensor, coupled to the externally-wearable patch.
Our THINK-Health 2018 consumer health/tech forecast is in the works, and #IOThealth will be on it. Early news out of CES Unveiled has shown us that digital health is certainly a growing category for #CES2018, and I’ll be on-the-Vegas-ground to explore the phenomenon. Expect the connected home and connected car to continue their blur toward the home-as-medical-home. FDA approval of Abilify MyCite is one point on this trajectory.




 Interviewed live on BNN Bloomberg (Canada) on the market for GLP-1 drugs for weight loss and their impact on both the health care system and consumer goods and services -- notably, food, nutrition, retail health, gyms, and other sectors.
Interviewed live on BNN Bloomberg (Canada) on the market for GLP-1 drugs for weight loss and their impact on both the health care system and consumer goods and services -- notably, food, nutrition, retail health, gyms, and other sectors. Thank you, Feedspot, for
Thank you, Feedspot, for  As you may know, I have been splitting work- and living-time between the U.S. and the E.U., most recently living in and working from Brussels. In the month of September 2024, I'll be splitting time between London and other parts of the U.K., and Italy where I'll be working with clients on consumer health, self-care and home care focused on food-as-medicine, digital health, business and scenario planning for the future...
As you may know, I have been splitting work- and living-time between the U.S. and the E.U., most recently living in and working from Brussels. In the month of September 2024, I'll be splitting time between London and other parts of the U.K., and Italy where I'll be working with clients on consumer health, self-care and home care focused on food-as-medicine, digital health, business and scenario planning for the future...