When I talk about patient support programs (PSPs), I’m most often focused on supporting peoples’ access to medicines due to costs, bolstering health literacy, and addressing health citizens’ risks of drivers of health that can be obstacles to optimal health outcomes (those challenging social determinants of health).
A new report from Deloitte on Intelligent post-launch patient support speaks to another crucial definition of patient support: post-market surveillance and patient safety.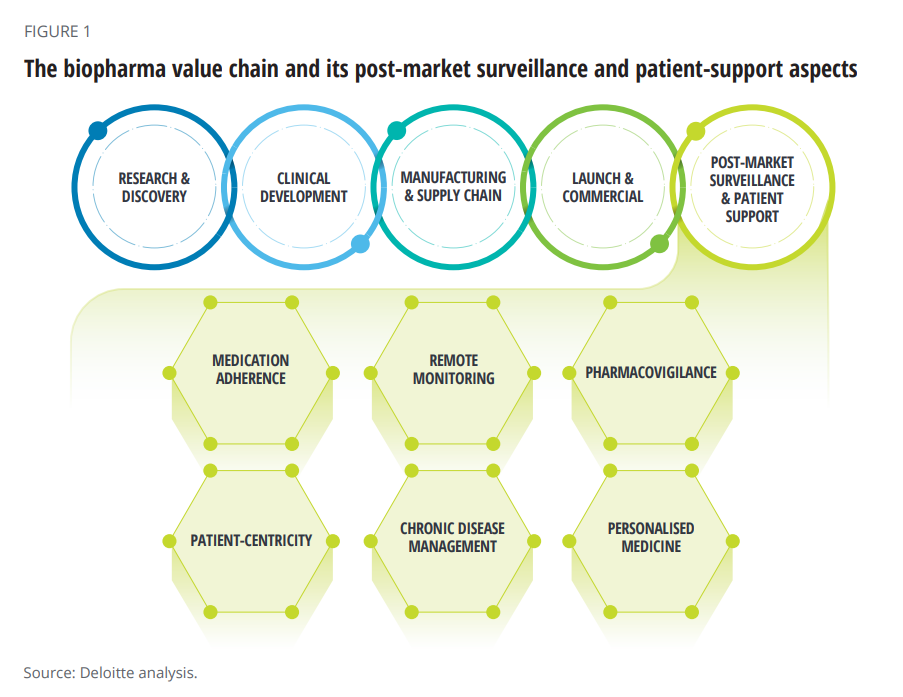
The paper’s central thesis is that improving patient support is a critical step in the biopharma value chain, illustrated in the first diagram from the report. Delivering value across the chain is underpinned by robust data that feeds artificial intelligence to deliver the “4Ps” that characterize contemporary medical care: those adjectives are personalized, preventive, predictive, and participatory.
You can see six patient support factors called out in the value chain: pharmacovigilance (PV) is the focus here: the ongoing monitoring of the safety and efficacy of an approved medicine after it is put on the market.
Deloitte identifies several trends that are reshaping the PV landscape, including,
- Growth in new medicines product portfolios and therapy areas that create more complexity, and
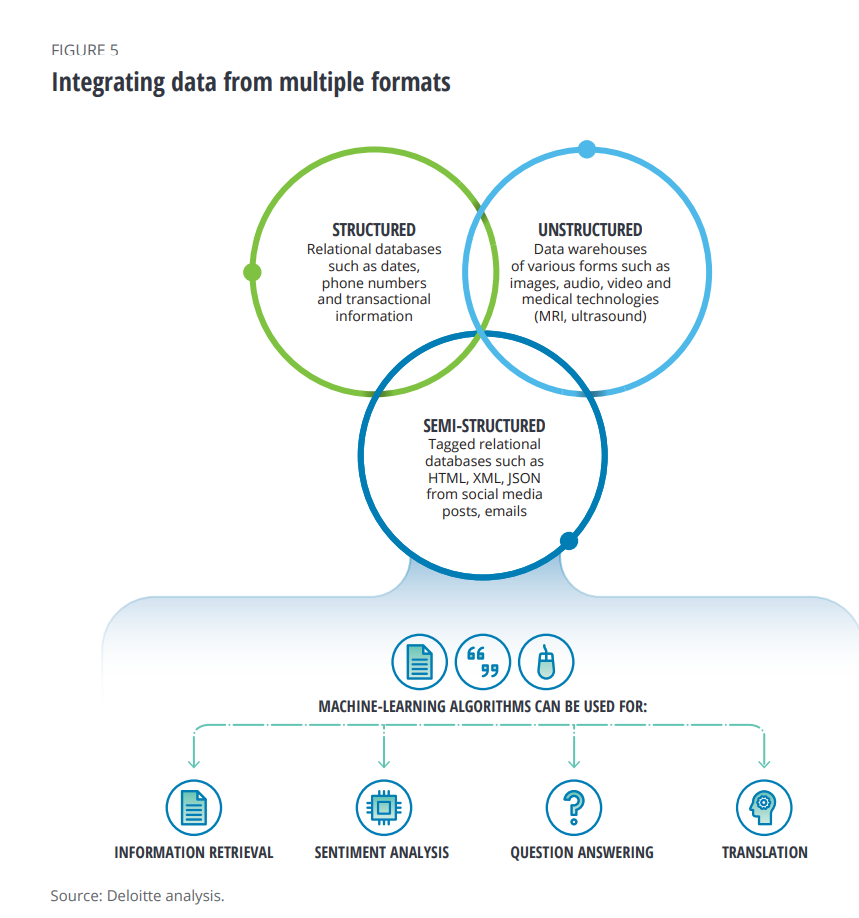
- New sources of information from wearable technologies and social media that are feeding AI algorithms, shown in the second diagram.
These novel data sources, beyond the traditional clinical trial information captured and medical claims data, can build better patient profiles based on real-world evidence and peoples’ daily lives.
But lack of interoperability has been a barrier to bringing the data together.
In the meantime, the FDA has received at least 2 million adverse events each year since 2018, which contribute to less-optimal patient outcomes — or, in the worst cases, deaths.
And the COVID-19 pandemic has raised the stakes for the biopharma industry to provide better patient support, Deloitte calls out.
It’s artificial intelligence technologies that can turbocharge PSP efforts, the paper advocates. Deloitte asserts that AI can improve PSPs’ success through,
- Better personalizing digital therapeutics
- Utilizing wearable technologies and remote monitoring among patients
- Processing diverse data
- Applying advanced analytics to better understand patient behavior
- Employing chatbots to bolster patient engagement, and
- Acknowledging and addressing patient concerns,
factors adapted from the research of Anita Osborn’s 10 steps to a successful patient support programme, published in 2016.
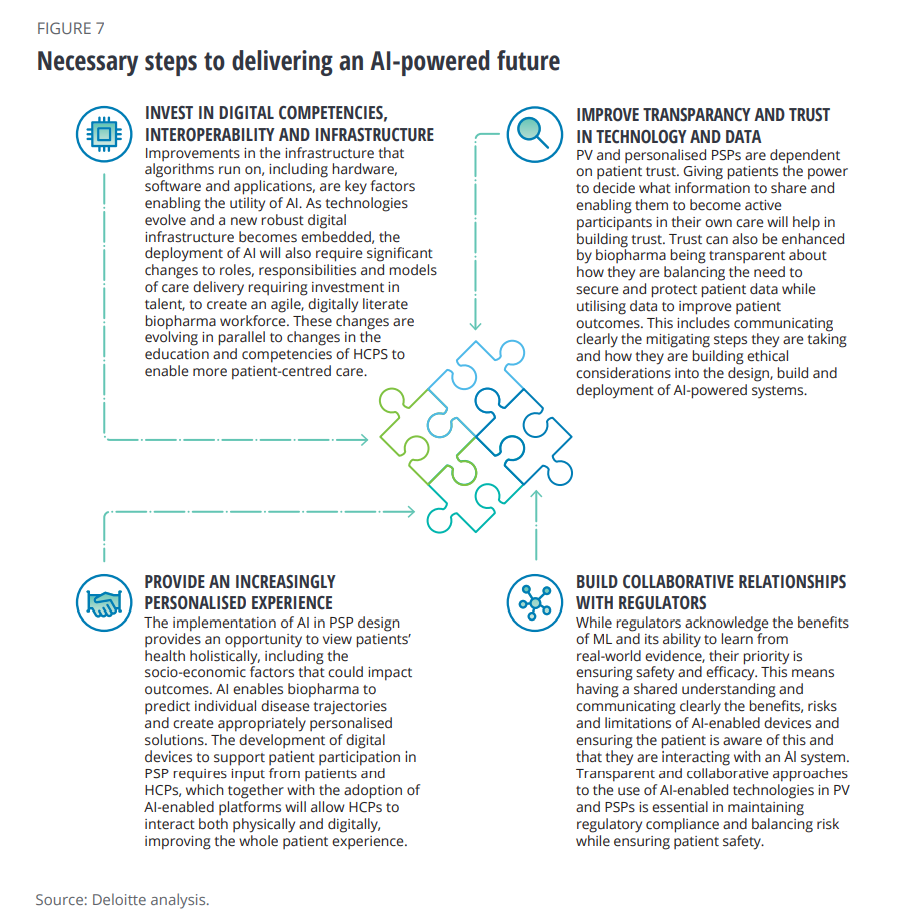
There are four steps toward realizing this vision, Deloitte outlines here in the third chart from the report:
- To invest in digital competencies, interoperability and infrastructure
- To improve transparency and trust and technology and data
- To provide a personalized experience
- To build collaborative relationships with regulators, balancing risk and ensuring patient safety.
This increasingly-personalize patient experience requires a trust framework between patients, providers, payers, and the biopharma industry. Deloitte rightly notes that the biopharma industry falls below other patient touchpoints when it comes to trust, identifying “four trust-building signals” in a 2021 report on overcoming the biopharma trust deficit:
- Start with humanity, ensuring that biopharma really cares for patients’ experience and well-being
- Mix in transparency, openly sharing information, motives and choices in plain understanding language
- Add in capability to serve up high-quality products and services that align with patients’ expectations, and
- Be reliable, consistent, and dependable across the patient journey.
“As biopharma embraces AI-powered digital transformation across the post-market ecosystem,” Deloitte writes, “the amount of patient data in the hands of these companies will exponentially increase…This requires biopharma to include ethical considerations into the design, build and deployment of AI-powered systems.”
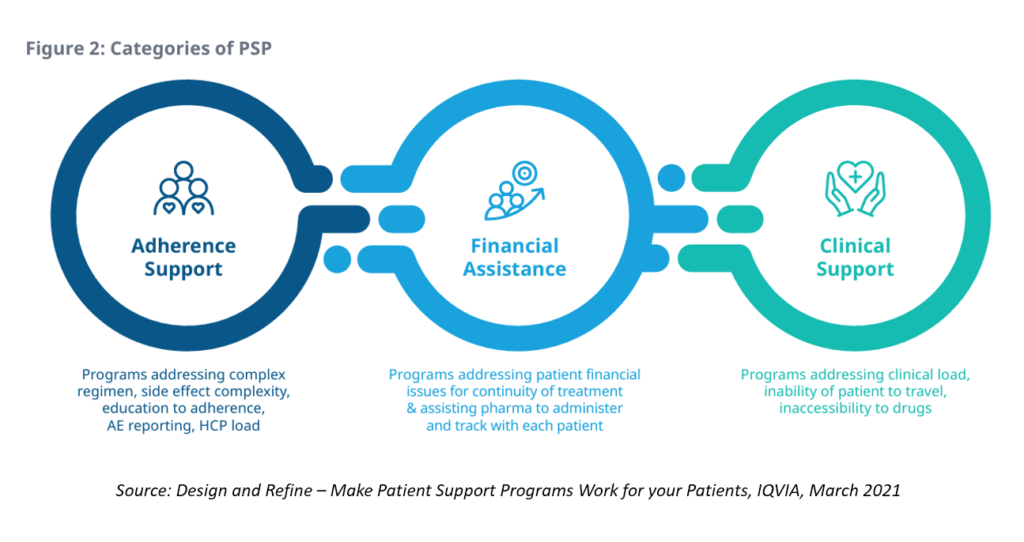
Health Populi’s Hot Points: We come full circle back to my more frequent focus on access to medicines and financial health for patient support programs, stated at the front of this post.
This diagram from IQVIA’s report, Design and Refine: Make Patient Support Programs Work for your Patients, incorporates the categories of PSPs which, in the eyes of patients, integrate across their personal journeys.
For patients to fully engaged with medicines in their treatment and self-care, trust must be embedded across these three categories (and others along the way). This is why I’m increasingly focused on ethics-by-design as part of the overall health-design process. Without trust, engagement will suffer, from initial adoption and persistence of therapies through to a patient’s financial health and, ultimately, helping to determine their outcomes — for better or worse.





 Interviewed live on BNN Bloomberg (Canada) on the market for GLP-1 drugs for weight loss and their impact on both the health care system and consumer goods and services -- notably, food, nutrition, retail health, gyms, and other sectors.
Interviewed live on BNN Bloomberg (Canada) on the market for GLP-1 drugs for weight loss and their impact on both the health care system and consumer goods and services -- notably, food, nutrition, retail health, gyms, and other sectors. Thank you, Feedspot, for
Thank you, Feedspot, for  As you may know, I have been splitting work- and living-time between the U.S. and the E.U., most recently living in and working from Brussels. In the month of September 2024, I'll be splitting time between London and other parts of the U.K., and Italy where I'll be working with clients on consumer health, self-care and home care focused on food-as-medicine, digital health, business and scenario planning for the future...
As you may know, I have been splitting work- and living-time between the U.S. and the E.U., most recently living in and working from Brussels. In the month of September 2024, I'll be splitting time between London and other parts of the U.K., and Italy where I'll be working with clients on consumer health, self-care and home care focused on food-as-medicine, digital health, business and scenario planning for the future...